The activation and conversion of carbon dioxide into added-value products is of economical and ecological importance.
To tackle this task, highly efficient catalysts are needed. Commercial catalysts however are typically energy demanding and often require high temperatures and pressures to achieve sufficient turnover.
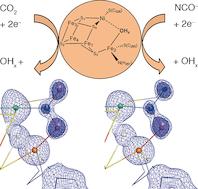
A highly efficient enzyme (Ni,Fe-dependent CO dehydrogenase, CODH) is found in a variety of anaerobic bacteria and catalyzes the initial activation and two-electron reduction of carbon dioxide (CO2) to carbon monoxide (CO) under ambient conditions. How CODHs activate carbon dioxide for turnover has been now been revealed in atomic details by UniCat scientists.
In their newest paper published in Angewandte Chemie, Jochen Fesseler, Jae-Hun Jeoung and Holger Dobbek (Humboldt-Universität zu Berlin) describe in unprecedented details how the activation of carbon dioxide (CO2) and its isoelectronic analogue NCO– is achieved at a complex biological metal center.
Carbon monoxide dehydrogenase (CODH) employs a [NiFe4S4] cluster (cluster C) to bind and activate carbon dioxide, which is subsequently split into carbon monoxide and water. By using x-ray crystallography, the authors were able to describe the clusters geometry with bound substrate carbon dioxide (CO2) and its inhibitor NCO– under turnover conditions at true-atomic resolution (dmin < 1.1 Å).
A comparison between the in-crystallo observed bond distances and angles with DFT calculations revealed, that the carbon dioxide moeity bound to cluster C is equivalent to a two-electron reduced carboxylate (CO22-). The bound carboxylate ligand with a µ2,η2 coordination geometry is stabilized by significant π-backbonding from cluster C and is arrested for the final protonating and bond-cleaving steps, leading to carbon monoxide and water.
According to the reviewers the study is of “very high importance”, a ranking received for less than 5% of the manuscripts in Angewandte Chemie. „Our insights will be of great help for synthetic inorganic and theoretical chemists“, explains Jochen Fesseler, lead author of the study. “By using our high-resolution structures as a blue print, complexes with comparable catalytic power could be developed for large-scale carbon dioxide conversions in the future.“
Citation:
Jochen Fesseler, Jae-Hun Jeoung, Holger Dobbek (2015): “How the [NiFe4S4]
Cluster of CO Dehydrogenase Activates CO2 and NCO−.“
Angew. Chem. Int. Ed. DOI: 10.1002/anie.201501778 (English)
Angew. Chem. DOI: 10.1002/anie.201501778 (German)