When an organic chemist needs strong reducing power he can turn to elemental alkali metals in an aprotic medium. When a physiological process requires an “energized” electron, it has to realize it without elemental metals as electron source and within an aqueous environment. One way how nature achieves this is shown in recent work of the UniCat groups of Peter Hildebrandt (TU Berlin) and Holger Dobbek (HU Berlin).
Cobalamin-containing methyltransferases depend on the nucleophilicity of Co(I) for abstracting a methyl-group from methylated folates or other methyl donors. When Co(I) looses one electron, the methyltransferase escapes the catalytic cycle and becomes inactive. Dedicated reactivating enzymes reduce the Co(II)-containing methyltransferase by enabling an otherwise unfavourable electron transfer. To do so the activating enzyme couples the electron transfer to the exergonic hydrolysis of ATP. How this coupling is achieved was hitherto unknown.
The groups of Hildebrandt and Dobbek revealed in a multidisciplinary approach using kinetics, spectroscopy and X-ray crystallography how redox-selective protein-protein and protein-cofactor interactions are orchestrated to achieve the difficult ATP-driven electron transfer.
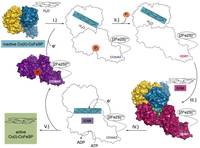
The activating enzyme termed RACo, acronym for the reductive activator of the corrinoid-iron/sulfur protein (CoFeSP), is part of the reductive acetyl-CoA pathway, in which CO2 is converted to activated acetic acid. In our publication in Nature Communications we show how RACo selectively binds Co(II) in inactive CoFeSP by an axial coordination, which is unfavorable with Co(I) in active CoFeSP. Electron transfer from the [2Fe2S]-cluster of RACo to Co(II) on CoFeSP is triggered by ATP-binding to RACo inducing large conformational changes that by breaking the axial coordination create a transient unstable fourfold-coordinated Co(II) ion that is prone to become reduced to Co(I). The results underline the importance of conformational gating for electron transfer and extend our knowledge about biological energy conversion.
More Information:
Research/Selected Research Highlights
Nature Communications | 5:4626 | DOI: 10.1038/ncomms5626
Published online 11 August 2014