New article by seven UniCat groups in Nature Chemical Biology
The collaborative effort of scientists from seven UniCat groups of all three Berlin universities and the Charité resulted in an article on the function of a novel iron-sulphur cluster species published in Nature Chemical Biology.
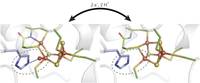
This unique [4Fe-3S] cofactor is located near the catalytic site of specialized hydrogen-converting biocatalysts, but its role in catalysis is not yet fully understood. Crystallographic, spectroscopic and computational data now provide evidence for redox-dependent structural and chemical transformations of this cofactor. This cluster morphing represents a crucial feature of particular biocatalysts that convert hydrogen in the presence of detrimental O2.
Nat. Chem. Biol. 2014; DOI: 10.1038/nchembio.1500
Published online on 6 April 2014.
For more information see Research\Selected Research Highlights.