Catalytic methods for the post-biosynthetic diversification of peptide antibiotics and proteins
Chemically accessible sidechains of amino acids in proteins and peptides are required if a desired functionality or label should be intruduced at a specific site of the protein. In more classical approaches these chemical modifications exploit reactive side chains of canonical amino acids (cAAs) such as the thiol group of Cys, the amine group of Lys or the carboxy group of Asp/Glu.
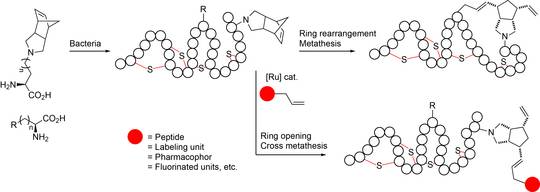
Alternatively, functional groups can be introduced into proteins and peptides by incorporating non-nanonical amino acids (ncAAs) in RPS and NRPS with appropriate bioorthogonal functionalities. Well known examples include azide groups or alkyne units for Cu(I) catalyzed alkyne-azide cycloadditions (‘click-chemistry’), halogenarene moieties for transition-metal catalyzed Sonogashira-, Suzuki-, or Heck-couplings, and also alkene units for less thoroughly explored olefin metathesis methodologies in water. Biocompatible olefin metathesis in water for introducing C-C couplings in peptides and proteins is of particular importance. Its development within this project represents a considerable challenge as it requires the application of water-soluble and water-stable catalysts.
Research goals
- Currently available methods for olefin metathesis on peptides and proteins are generally limited to special solvent-resistant species since the reactions take place in aqueous solvent mixtures. To overcome these limits biocompatible olefin metathesis methodologies on the basis on Blechert-Hoveyda catalysts will be developed and tailored for post-biosynthetic reactions.
- Model reactions at the example of peptides accessible by automated synthesis to gain proof-of-principle data will be performed. Subsequently, these procedures will be extended to expressed ribosomally and non-ribosomally peptides and proteins, respectively containing ncAAs and subjected to bioorthoganal modifications.
- For controlling and timing of specific peptide bioactivities the ability of light-induced activation represents an elegant access towards analyzing the mechanism and dynamics of the molecular processes associated with the interactions of the peptide with the biological targets, i.e. biological membranes. To probe these processes by time-resolved spectroscopic techniques, post-biosynthetic conjugation of photoswitches to ncAAs will be designed.