On the design, development and operation of an energy efficient CO2 removal for the oxidative coupling of methane in a miniplant scale
The aim of this research is the demonstration of the technical feasibility of the OCM process, including product recovery in a miniplant scale.
Steffen Stünkel, Daniel Illmer, Andreas Drescher, Reinhard Schomäcker, Günter Wozny
Applied Thermal Engineering 2011
Published online October 25, 2011
doi: 10.1016/j.applthermaleng.2011.10.035
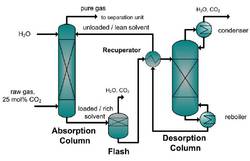
In this article the gas treatment of the reaction product gas is discussed as a key part of the production chain from raw material to the product.
In the OCM process the CO2 is treated as an unwanted by-product or waste. Nevertheless, the energy and the costs requirements for the whole OCM process are crucial for industrial applicability and must be kept as low as possible to operate economically. Therefore different separation methods are examined and several process alternatives are investigated. In this article the energy saving of a hybrid separation process based on a membrane-absorption process for the CO2 capture of the OCM process is presented and proved experimentally in miniplant scale. In a case study of the OCM process the development of an integrated gas treatment process for CO2 capture in the miniplant scale is presented and experimental results are discussed.
Lithium as a Modifier for Morphology and Defect Structure of Porous Magnesium Oxide Materials Prepared by Gel Combustion Synthesis
Ulyana Zavyalova, Gisela Weinberg, Wiebke Frandsen, Frank Girgsdies, Thomas Risse, Klaus Peter-Dinse, Robert Schloegl, Raimund Horn
ChemCatChem 2011, 3, 1179-1788.
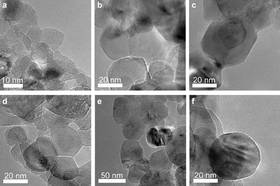
Defect rich MgO nanocrystals arranged in a hierarchic three-dimensional pore network were synthesized by using gel combustion synthesis (GCS).By adding Li to the combustion precursor, Li-induced changes in the morphology and defect structure of MgO could be studied systematically.
At low Li loadings (up to 1 wt %), the three-dimensional pore network was resistant to temperatures up to 800 °C, even though the primary MgO nanoparticles had changed their morphology from on average 8 nm size {100} terminated nanocubes to up to 250 nm large complex polyhedral, exposing more and more {111} facets.
At higher Li loadings, the primary MgO particles grow even further, to up to 500 nm, causing the three-dimensional pore network to collapse. After describing the GCS method, detailed structural characterizations of all of the materials synthesized were conducted by means of XRD, BET and pore size analysis, and electron microscopy. IR and thermogravimetric mass spectroscopy (TG-MS) in combination with XRD were used to investigate the formation and decomposition of carbonate species during synthesis and calcination.
Diffuse reflectance UV/Vis (DR-UV/Vis) spectroscopy was used to characterize surface defects, such as low coordinated O2− ions at edges, corners, and kinks of the MgO surface. Bulk defects were studied by using electron paramagnetic resonance (EPR) spectroscopy. Morphology and defect concentration of the Li/MgO materials were found to be strongly dependent on the fuel-to-oxidizer ratio used in the combustion synthesis, the Li concentration, and the calcination atmosphere.
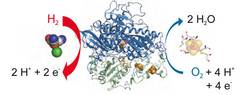
The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre
New results on biological hydrogen conversion: structure of a microbial hydrogen engine
Johannes Fritsch, Patrick Scheerer, Stefan Frielingsdorf, Sebastian Kroschinsky, Bärbel Friedrich, Oliver Lenz and Christian M. T. Spahn
Nature 2011, doi: 10.1038/nature10505
Published online October 16, 2011
Hydrogenases are abundant enzymes that catalyse the reversible interconversion of H2 into protons and electrons at high rates. Those hydrogenases maintaining their activity in the presence of O2 are considered to be central to H2-based technologies, such as enzymatic fuel cells and for light-driven H2 production. Despite comprehensive genetic, biochemical, electrochemical and spectroscopic investigations, the molecular background allowing a structural interpretation of how the catalytic centre is protected from irreversible inactivation by O2 has remained unclear.
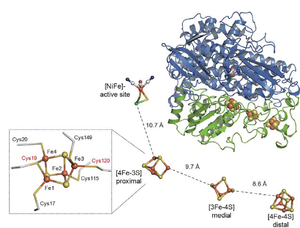
Here we present the crystal structure of an O2-tolerant [NiFe]-hydrogenase from the aerobic H2 oxidizer Ralstonia eutropha H16 at 1.5 Å resolution. The heterodimeric enzyme consists of a large subunit harbouring the catalytic centre in the H2-reduced state and a small subunit containing an electron relay consisting of three different iron-sulphur clusters. The cluster proximal to the active site displays an unprecedented [4Fe-3S] structure and is coordinated by six cysteines.
Hydrogenase Spills Secret
A German team led by Oliver Lenz of Humboldt University and Christian M. T. Spahn of Charité University Hospital, both in Berlin, and ... detected a cluster never before seen in an enzyme—four iron atoms and three sulfurs.
Chemical & Engineering News, 89(43), October 24, 2011
Lessons from isolable nickel(I) precursor complexes for small molecule activation
Shenglai Yao and Matthias Driess
Acc. Chem. Res. 2011, doi 10.1021/ar200156r
Published online August 29, 2011
Small-molecule activation by transition metals is essential to numerous organic transformations, both biological and industrial. Creating useful metal-mediated activation systems often depends on stabilizing the metal with uncommon low oxidation states and low coordination numbers. This provides a redox-active metal center with vacant coordination sites well suited for interacting with small molecules.
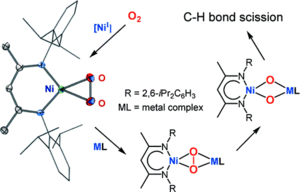
Monovalent nickel species, with their d9 electronic configuration, are moderately strong one-electron reducing agents that are synthetically attractive if they can be isolated. They represent suitable reagents for closing the knowledge gap in nickelmediated activation of small molecules. Recently, the first strikingly stable dinuclear β-diketiminate nickel(I) precursor complexes were synthesized, proving to be suitable promoters for small-molecule binding and activation. They have led to many unprecedented nickel complexes bearing activated small molecules in different reduction stages.
In this Account, we describe selected achievements in the activation of nitrous oxide (N2O), O2, the heavier chalcogens (S, Se, and Te), and white phosphorus (P4) through this β-diketiminatonickel(I) precursor species. We emphasize the reductive activation of O2, owing to its promise in oxidation processes. The one-electron-reduced O2 activation product, that is, the corresponding β-diketiminato-supported Ni-O2 complex, is a genuine superoxonickel(II) complex, representing an important intermediate in the early stages of O2 activation. It selectively acts as an oxygen-atom transfer agent, hydrogen-atom scavenger, or both towards exogenous organic substrates to yield oxidation products.
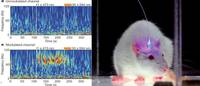
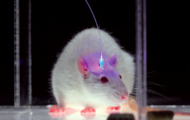
Neocortical excitation/inhibition balance in information processing and social dysfunction
Ofer Yizhar, Lief E. Fenno, Matthias Prigge, Franziska Schneider, Thomas J. Davidson, Daniel J. O’Shea, Vikaas S. Sohal, Inbal Goshen, Joel Finkelstein, Jeanne T. Paz, Katja Stehfest, Roman Fudim, Charu Ramakrishnan, John R. Huguenard, Peter Hegemann & Karl Deisseroth
Nature, 2011, doi: 10.1038/nature10360, Published online July 27, 2011
Severe behavioural deficits in psychiatric diseases such as autism and schizophrenia have been hypothesized to arise from elevations in the cellular balance of excitation and inhibition (E/I balance) within neural microcircuitry. This hypothesis could unify diverse streams of pathophysiological and genetic evidence, but has not been susceptible to direct testing.
Here we design and use several novel optogenetic tools to causally investigate the cellular E/I balance hypothesis in freely moving mammals, and explore the associated circuit physiology. Elevation, but not reduction, of cellular E/I balance within the mouse medial prefrontal cortex was found to elicit a profound impairment in cellular information processing, associated with specific behavioural impairments and increased high-frequency power in the 30–80 Hz range, which have both been observed in clinical conditions in humans.
Consistent with the E/I balance hypothesis, compensatory elevation of inhibitory cell excitability partially rescued social deficits caused by E/I balance elevation. These results provide support for the elevated cellular E/I balance hypothesis of severe neuropsychiatric disease-related symptoms.
Synthetic manganese–calcium oxides mimic the water-oxidizing complex of photosynthesis functionally and structurally
Ivelina Zaharieva, M. Mahdi Najafpour, Mathias Wiechen, Michael Haumann, Philipp Kurz and Holger Dau
Energy Environ. Sci. 2011, 4, 2400-2408
Holger Dau and coworker present new atomistic models of the structures of the manganese–calcium oxides, which are derived from results of X-ray absorption spectroscopy. The presented results have important implications for both biological water oxidation and the design of catalysts for solar fuel production.
In the worldwide search for sustainable energy technologies, water oxidation by abundant low-cost materials is of key importance. In nature, this process is efficiently catalyzed by an intricate manganese–calcium (Mn4Ca) complex bound to the proteins of photosystem II (PSII). Recently synthetic manganese–calcium oxides were found to be active catalysts of water oxidation but at the atomic level their structure has remained elusive.
To investigate these amorphous catalysts, extended-range X-ray absorption spectroscopy (XAS) at the K-edges of both manganese and calcium was performed. The XAS results reveal striking similarities between the synthetic material and the natural Mn4Ca complex.
The oxidation state of manganese in the active oxides was found to be close to +4, but MnIII ions are present as well at a level of about 20%. Neighboring Mn ions are extensively interconnected by two bridging oxygens, a characteristic feature of layered manganese oxides. However, the oxides do not exhibit long-range order, as opposed to canonical, but catalytically inactive MnIII- or MnIV-oxides. Two different Ca-containing motifs were identified. One of them results in the formation of Mn3CaO4 cubes, as also proposed for the natural paragon in PSII.
Other calcium ions likely interconnect oxide-layer fragments. We conclude that these readily synthesized manganese–calcium oxides are the closest structural and functional analogs to the native PSII catalyst found so far. Evolutionary implications are considered. From the differences to inactive manganese oxides, we infer structural features facilitating the catalysis of water oxidation in both the protein-bound Mn4Ca complex of PSII and in the synthetic oxides.
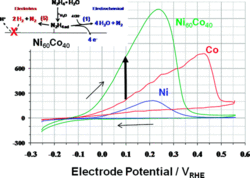
Noble Metal-Free Hydrazine Fuel Cell Catalysts: EPOC Effect in Competing Chemical and Electrochemical Reaction Pathways
Jean Sanabria-Chinchilla, Koichiro Asazawa, Hirohisa Tanaka, and Tomokazu Sakamoto, Koji Yamada, Peter Strasser
J. Am. Chem. Soc. 2011, 133, 5425-5431
We report the discovery of a highly active Ni-Co alloy electrocatalyst for the oxidation of hydrazine (N2H4) and provide evidence for competing electrochemical (faradaic) and chemical (nonfaradaic) reaction pathways. The electrochemical conversion of hydrazine on catalytic surfaces in fuel cells is of great scientific and technological interest, because it offers multiple redox states, complex reaction pathways, and significantly more favorable energy and power densities compared to hydrogen fuel.
Structure-reactivity relations of a Ni60Co40 alloy electrocatalyst are presented with a 6-fold increase in catalytic N2H4 oxidation activity over today’s benchmark catalysts. We further study the mechanistic pathways of the catalytic N2H4 conversion as function of the applied electrode potential using differentially pumped electrochemical mass spectrometry (DEMS). At positive overpotentials, N2H4 is electrooxidized into nitrogen consuming hydroxide ions, which is the fuel cellrelevant faradaic reaction pathway.
In parallel, N2H4 decomposes chemically into molecular nitrogen and hydrogen over a broad range of electrode potentials. The electroless chemical decomposition rate was controlled by the electrode potential, suggesting a rare example of a liquid-phase electrochemical promotion effect of a chemical catalytic reaction (“EPOC”). The coexisting electrocatalytic (faradaic) and heterogeneous catalytic (electroless, nonfaradaic) reaction pathways have important implications for the efficiency of hydrazine fuel cells.
A unique iron-sulfur cluster is crucial for oxygen tolerance of a [NiFe]-hydrogenase
Tobias Goris, Annemarie F Wait, Miguel Saggu, Johannes Fritsch, Nina Heidary, Matthias Stein, Ingo Zebger, Friedhelm Lendzian, Fraser A Armstrong, Bärbel Friedrich & Oliver Lenz
Nat. Chem. Biol., Published online: 09 March 2011.
doi:10.1038/nchembio.555
Hydrogenases are essential for H2 cycling in microbial metabolism and serve as valuable blueprints for H2-based biotechnological applications. However, most hydrogenases are extremely oxygen sensitive and prone to inactivation by even traces of O2. The O2-tolerant membrane-bound [NiFe]-hydrogenase of Ralstonia eutropha H16 is one of the few examples that can perform H2 uptake in the presence of ambient O2.
Here we show that O2 tolerance is crucially related to a modification of the internal electron-transfer chain. The iron-sulfur cluster proximal to the active site is surrounded by six instead of four conserved coordinating cysteines. Removal of the two additional cysteines alters the electronic structure of the proximal iron-sulfur cluster and renders the catalytic activity sensitive to O2 as shown by physiological, biochemical, spectroscopic and electrochemical studies.
The data indicate that the mechanism of O2 tolerance relies on the reductive removal of oxygenic species guided by the unique architecture of the electron relay rather than a restricted access of O2 to the active site.
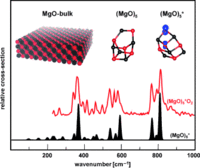
Structural diversity and flexibility of MgO gas-phase clusters
Karolina Kwapien, Marek Sierka, Jens Döbler, Joachim Sauer, Marko Haertelt, André Fielicke, and Gerard Meijer
Angew. Chem. Int. Ed. 2011, 50, 1716
Only one structure type is known for bulk MgO. In contrast, quantum chemical calculations combined with infrared multiple photon dissociation experiments demonstrate that neutral and cationic gas-phase clusters of MgO display unusual structural diversity and flexibility (see picture).
Method of the year
Nature Methods' choice of Method of the Year 2010 is optogenetics for its capacity to control cell function with light. Peter Hegemann's channelrhodopsins play a major role:
A series of articles and a video describe how optogenetics has revolutionized the way experiments are conducted in neuroscience and showcase the potential the method has for the study of many signaling pathways in cell biology.