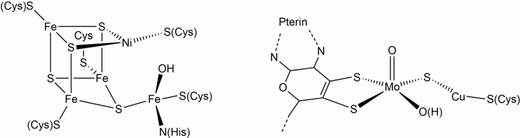
Molecular models for carbon monoxide dehydrogenases (CODHs)
In addition to the complete and partial reduction of CO2 studied in D2-1 and D2-2, this project focuses on the conversion of CO/CO2, inspired by natural enzymatic processes. Research is devoted to the synthesis of functional model compounds for mimicking and analyzing the active sites of carbon monoxide dehydrogenases (CODHs).
CODHs are metalloenzymes containing [NiFe] or [CuMo] active sites, where CO is oxidised to CO2 (see Fig. ). Furthermore, various bacteria use CO also for the synthesis of acetyl-Co-enzyme A. As CO oxidation to CO2 by [NiFe] CODHs is reversible, these enzymes also ensure CO supply for these purposes.
Chemical challenges are the synthesis of functional models for the elucidation of elementary steps of CO2 binding and subsequent reduction.
The proposed mechanistic model for the [NiFe]-containing CODH, involving five steps in the catalytic cycle, raises key questions concerning, for instance, the oxidation state of Ni in the reduced form of the cofactor (Ni0, NiI, or NiII), the nature of intermediate states formed during the two-electron reduction of CO2, the electronic influence of the [Fe3S4] cluster on the Ni centre, the presence of a Ni hydride in the reduced state, and the role of the exocyclic Fe sites during reduction.
Research goals
Functional, homo- and heterobimetallic [NiNi] and [NiFe] compounds will be synthesized and probed as M,M´-CODH model systems. Likewise, the synthesis of functional models of [CuMo]-containing CODHs is carried out to shed light on molecular details of the catalytic cycle of CO oxidation mediated by these enzymes.