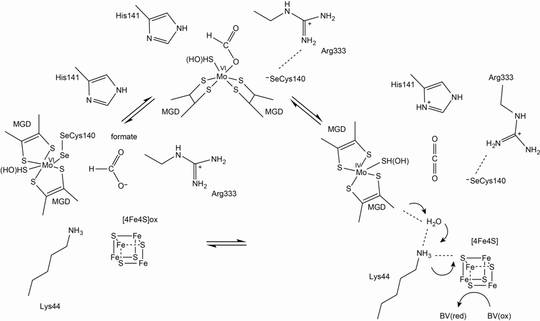
Formate dehydrogenase
Formate dehydrogenases (FDHs) catalyse, in general, the oxidation of formate to CO2 and H+. However, some FDHs can act as a CO2 reductase, catalysing the reverse reaction. FDHs are found in anaerobic bacteria and archaea, in addition to facultative anaerobic or aerobic bacteria and yeasts.
These enzymes are highly diverse and employ different cofactors such as the molybdenum cofactor (Moco), [FeS] clusters and FAD, or, in case of the yeast enzyme, can even exert the catalytic function without any cofactor. Some enzymes include tungsten in place of molybdenum at the active site. We will mainly focus on the characterisation of two different FDHs: anaerobic Se-Cys-containing FDHs and oxygen-tolerant FDHs from bacteria.
Research goals
- We will analyse the catalytic mechanism of FDHs, attempting to clarify various unresolved questions as to the nature of the Mo ligands and the parameters that control the directionality of the catalytic process.
- The enzyme with a cysteine instead of the selenocysteine (SeCys) is 1000-fold less active, but still exhibits some catalytic activity. We will express the Se-Cys E. coli FdhF, in addition to a variant in which the Se-Cys140 is replaced by a Cys, establishing a homologous expression system in E. coli. Both Cys and SeCys protein variants will be characterised in terms of their catalytic mechanism.
- In addition, we will try to generate a tungsten(W)-substituted enzyme of FDH-H. In general, W-containing isoenzymes from the class of molybdenum- and tungsten enzymes are thermally more stable and possess a redox potential lower by 300 - 400 mV compared to the Mo-analogue. Since the W-variant will catalyse reactions with a lower redox potential, we will try to generate a novel „CO2 reductase“ from FDH-H
- A wide range of techniques will be applied to determine structures of enzymes by X-ray crystallography, to elucidate the nature of the various states of the catalytic cycles and the dynamics of the processes by IR, RR, and EPR spectroscopy, and to monitor the electron transfer processes by means of CV. The experimental studies will be accompanied by theoretical investigations carried out to support spectra interpretation and the analysis of the redox properties and electron transfer.