Mimicking acetyl-coenzyme A synthase
The carbon monoxide dehydrogenase acetyl Co-enzyme A synthase (CODH/ACS) is a bifunctional enzyme, which couples the reduction of CO2 to the conversion of CO, a methyl group and co-enzyme A (HSCoA) to give acetyl co-enzyme A (CH3C(O)SCoA).
Recent advances in X-ray crystallography have significantly contributed to the understanding of the active site, revealing two Ni centres linked to a [Fe4S4] cluster. Mechanistic questions that remain unresolved include the binding sequence, the electronic structure of the reduced di-nickel site (NiINiII or Ni0NiII or even a higher reduced state), and the influence of the [Fe4S4] cluster.
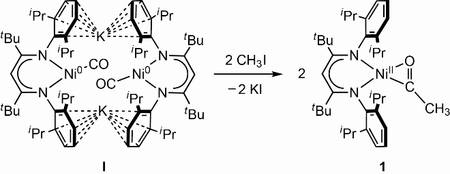
We have recently established the first example of a Ni-CO compound that is converted into a Ni-C(O)-CH3 complex by transfer of CH3+, indicating that CO binding may be the first event in the catalytic cycle, especially since subsequent C-S bond formation could be realised, too.
Research goals
Future studies will aim at elucidating the dependence of the reactivity on the oxidation state of the Ni centre and the electronic structure of its surroundings, i.e., iron/sulphur or other appendices providing reducing equivalents.
Furthermore, novel functional dinuclear [NiNi] complexes will be synthesised and investigated.