A2 Tailoring metal support interactions
State of the art
Transition metal particles (≈ 3-8nm diameter) supported on metal oxides represent the largest class of solid catalysts used in industrial processes. During the lifetime of a catalyst particle growth of active particles into larger, aggregates is regarded to be a major, inevitable problem. Aggregation driven by Ostwald ripening is influenced by the strength of the metal-support interaction. It is known that interactions with small inorganic ligands, e.g. Cl-, OH- facilitate high degrees of dispersion. It is desirable to be able to chemically redisperse agglomerated nanoparticles and thus rejuvenate catalysts in situ.
On an industrial scale, acetate and citrate ligands have been used in this fashion. Yet the con-cept of using molecular auxiliaries to redisperse metallic catalysts, despite its attractiveness, is far from being understood or established. Furthermore, the competition between intentionally added ligands and the unintentionally present terminating groups from the support opens up the possibility of the rational design of metal-support phenomena, if the strength and speci-ficity of the interaction between metal nanoparticles and ligand can be tailored.
 |
Research goals
Information on strength, type and specificity of the interactions of metals or metal particles with appropriate ligands is being obtained by combining differently scaled model systems similar to the approach introduced in Research Field A1 . We are investigating how the presence of such ligands affects the morphology, stability, mobility and reactivity of metal clusters on oxide surfaces and in the gas phase. Mobile (gold) and less mobile (copper) metals have been selected as model systems.
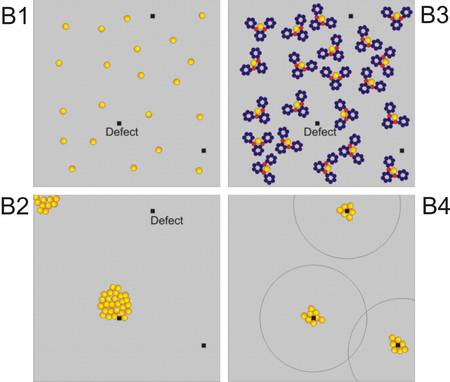
The strategy may be schematically displayed as shown in Fig. 1: at room or elevated tempera-tures metal atoms are diffusing constantly across the surface between large particles (B1) which leads to Oswald ripening (B2). When exposed to molecules which are able to capture those diffusing atoms, small ligand-stabilized clusters will be dispersed across the surface, with a distribution basically determined by the radius of diffusion of the metal atoms (B3). If it is possible then to release the clusters from the ligand a redispersion of the catalyst would result (B4).
Research achievements
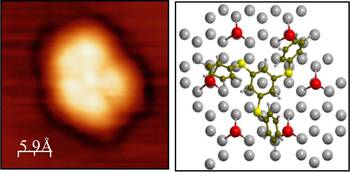
A specific mid-term objective in controlling redispersion was to study metal support interac-tions and specifically to investigate innovative approaches to this process. During operation of a supported metal catalyst, the diffusion process is controlled by metal substrate interactions and may be influenced chemically by support modifications [1]. Also the charge on the metal particles has been shown to play an important role [2; 3]. These phe¬nomena have been studied at atomic resolution to identify positively and negatively charged as well as neutral ligand stabilized or free Au clusters at the surface [4, 5, 6].
 |
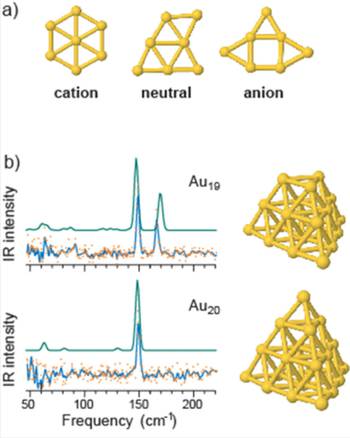
It was particularly instructive to refer to gas phase studies on Au clusters at various charge states, among which the structure of neutral clusters could be characterized for the first time [7]. Fig. 3 shows that the experimentally determined structures of gold clusters containing seven Au atoms vary for the different change states. Also, a comparison of experimental and calculated IR spectra for neutral Au19 and Au20 is presented in Fig. 3.
 |
To address the active control of particle sizes for the catalyst function, the initial size distribu-tion has to be recovered from a catalyst that has undergone Ostwald ripening. In this respect, an approach could be established that is based on Au nanoparticles coated by tailored organic sulfur ligands, which capture diffusing metal atoms and stabilise small metal particles. This process is thermodynamically favored as revealed by experimental and theoretical studies on well-defined Au(111) surfaces. Ongoing research aims at narrowing the size distribution of the Au particles and extending the model studies to magnesia supports.
References
- E. Carrasco, M. A. Brown, M. Sterrer, H. J. Freund, K. Kwapien, M. Sierka, J. Sauer; Thickness-dependent hydroxylation of MgO(001) thin film; J. Phys. Chem. C 2010, 114, 18207-18214.
- X. Lin, B. Yang, H. M. Benia, P. Myrach, M. Yulikov, A. Aumer, M. Brown, M. Ster-rer, O. Bondarchuk, E. Kieseritzky, J. Rocker, T. Risse, H. Gao, N. Nilius, H.-J. Freund; Charge-mediated adsorption behavior of CO on MgO-supported Au clusters; J. Am. Chem. Soc. 2010, 132, 7745-7749.
- T. Risse, S. Shaikhutdinov, N. Nilius, M. Sterrer, H. J. Freund; Gold supported on thin oxide films: From single atoms to nanoparticles; Acc. Chem. Res. 2008, 41, 949-956.
- N. Nilius, M. V. Ganduglia-Pirovano, V. Brazdova, M. Kulawik, J. Sauer, H. J. Freund; Counting electrons transferred through a thin alumina film into Au chains; Phys. Rev. Lett. 2008, 100, 096802 [4 pages].
- N. Nilius, M. V. Ganduglia-Pirovano, V. Brazdova, M. Kulawik, J. Sauer, H. J. Freund; Electronic properties and charge state of gold monomers and chains adsorbed on alumina thin films on NiAl(110); Phys. Rev. B 2010, 81, 045422 [7 pages].
- G. H. Simon, T. König, H.-P. Rust, M. V. Ganduglia-Pirovano, J. Sauer, M. Heyde, H. J. Freund; Imaging of individual adatoms on oxide surfaces by dynamic force mi-croscopy; Phys. Rev. B 2010, 81, 073411 [4 pages].
- P. Gruene, D. M. Rayner, B. Redlich, A. F. G. van der Meer, J. T. Lyon, G. Meijer, A. Fielicke; Structures of neutral Au7, Au19, and Au20 clusters in the gas phase; Science 2008, 321, 674-676.
Project team and expertise
| Prof. Dr. Siegfried Blechert (TU Berlin) | Homogeneous catalysis, organic synthesis, natural product synthesis |
| Prof. Dr. Hans-Joachim Freund (FHI) | Spectroscopy and reactivity of metal clusters on oxide films |
| Prof. Dr. Gerard Meijer (FHI) | IR spectroscopy of metal and metal oxide clusters and their complexes |
| Prof. Dr. Joachim Sauer (HU Berlin) | Quantum mechanical studies of reaction mechanisms on surfaces and in clusters |
| Prof. Dr. Matthias Scheffler (FHI) | Electronic structure theory, density functional theory, first-principles statistical mechanics |
Former team members | |
| Dr. Karsten Reuter (FHI) | Density functional theory, first-principles statistical mechanics and kinetic Monte Carlo |
| Prof. Dr. Robert Schlögl (FHI) | In situ studies of model and real catalysts, synthesis and functional testing |
| Prof. Dr Ludger Wöste (FU Berlin) | Gas-phase reactivity of small metal clusters |


