A4 Consecutive catalysis for fine chemical synthesis
Homogeneous catalysis provides indispensable tools for the preparation of a variety of important organic compounds, e.g. pharmaceutical intermediates, and is primarily target-oriented. Modern synthesis aims at reducing the number of steps, increasing energy efficiency, covering raw material consumption and avoiding by-products and waste. A promising concept is the use of tandem reactions that involve the sequential utilisation of catalytic transformations. The main targets with this research field are:
- (a) Search for innovative catalysts suitable for new C-X coupling reactions (e.g. X = C, N, O) affording organic target molecules including enantiopure compounds of significant impact in pharmaceutical industry.
- (b) Coupling of different catalytic steps into one consecutive chain, development of tandem reactions including olefin metathesis.
- (c) Linking homogenous and heterogeneous catalysis.
Results/Achievments

(a) On the field of catalyst design considerable progress could be achieved. A highly reactive rhodium (I)-boryl complex turned out as a useful tool for C-H bond activation and catalytic C-F bond borylation [1]. Different zinc-catalysts for the reduction of ketones have been developed [2, 3]. New zinc-complexes have also been applied for catalytic hydroamination. The outstanding property in comparison to other metal catalysts is their excellent tolerance towards functional groups [4, 6]. Several novel ligands for enantioselective ruthenium-catalyzed olefin metathesis allow very efficient and highly selective C-C-bond formation. The Ru-complexes outer perform all other know Ru-catalysts concerning selectivity, reactivity and stability [7, 8].
(b) In the specific field of consecutive catalysis diverse catalytic reactions were developed. Amongst others, tandem reactions yielding chiral oxygenated heterocycles and sequential Zn-Ru catalysis were realized. [9]
(c) Strong added values are visible in the cooperation A4 with A3 (Antonietti-Blechert-Thomas and Blechert-Thomas). Polymerizable chiral phosphoric acids (organocatalysts) developed by the Blechert group could latterly be used as building blocks by the group of Thomas to generate mesoporous chiral organic materials with catalytic activity. The in A4 realized test reactions showed a much higher enantioselectivity compared to the homogeneous catalysts, which is a considerable benefit next to the catalyst’s straightforward recovery (see A3 ).
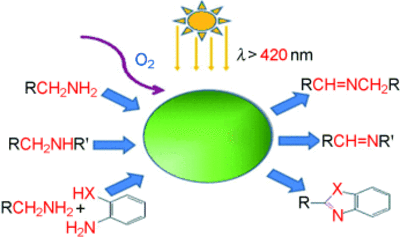
The cooperation with the group of Antonietti (A3) resulted in significant progress regarding highly selective oxidation methods. Utilization of graphitic carbon nitrides (C3N4) enables aerobic oxidation of alcohols and amines under very mild conditions and allow the synthesis of different heterocycles by a simple reaction cascade [10, 11].
Within a project focussing on dual, heterogeneous catalysis and NHC-catalysis (N-heterocyclic carbenes) a new methodology of highly selective transformations from aldehydes to acids, ester and amides, which are difficult to obtain by classical methods, has been developed successfully. The research on applications of graphitic carbon nitrides for environmentally benign synthesis is a fast growing field. In this concern the cluster is holding an international leading position.
 |
Most important publications
- A Highly Reactive Rhodium(I)–Boryl Complex as a Useful Tool for C-H Bond Activation and Catalytic C-F Bond Borylation, Michael Teltewskoi, Julien A. Panetier, Stuart A. Macgregor, and Thomas Braun, Angewandte Chemie 2010, 49, 3947-3951.
- Facile and Efficient Reduction of Ketones in the Presence of Zinc Catalysts Modified by Phenol Ligands, Stephan Enthaler, Björn Eckhardt, Shigeyoshi Inoue, Elisabeth Irran, and Matthias Driess, Chem. Asian J. 2010, 5, 2027-2035.
- High Efficiency in Catalytic Hydrosilylation of Ketones with Zinc based Precatalysts featuring hard and soft Tridentate O,S,O-Ligands, N. A. Marinos, S. Enthaler, M. Driess, ChemCatChem 2010, 2, 846-853.
- Electronic modification of an aminotroponiminate zinc complex leading to an increased reactivity in the hydroamination of alkenes, Maximilian Dochnahl, Karolin Loehnwitz, Jens-Wolfgang Pissarek, Peter W. Roesky, Siegfried Blechert, Dalton Transactions 2008, 21, 2844-2848.
- Intramolecular hydroamination with homogeneous zinc catalysts: Evaluation of substituent effects in N,N’-disubstituted aminotroponiminate zinc complexes, Maximilian Dochnahl, Karolin Loehnwitz, Jens-Wolfgang Pissarek, Mustafa Biyikal, Sabrina R. Schulz, Sebastian Schön, Nils Meyer, Peter W. Roesky, Siegfried Blechert, Chem. A Eur.J. 2007, 13, 6654-6666.
- Zinc-zinc bonded decamethyldizincocene Zn2(η5-C5Me5)2 as catalyst for the inter- and intramolecular hydroamination reaction, Anja Lühl, Hari Pada Nayek, Siegfried Blechert, Peter W. Roesky, Chemical Communications 2011, 47, 8280-8282.
- Highly Active Chiral Ruthenium-Based Metathesis Catalysts through a Monosubstitution in the N-Heterocyclic Carbene, Sascha Tiede, Anke Berger, David Schlesiger, Daniel Rost, Anja Lühl, and Siegfried Blechert, Angewandte Chemie 2010, 49, 3972-3975.
- A Novel Ligand for the enantioselective Ruthenium-catalyzed Olefin Metathesis, Axel Kannenberg, Daniel Rost, Stefan Eibauer, Sascha Tiede, Siegfried Blechert, Angewandte Chemie, International Edition, 3299-3302.
- Zinc-Catalyzed Domino Hydroamination-Alkyne Addition, Mustafa Biyikal, Marta Porta, Peter W. Roesky, Siegfried Blechert, Adv. Synth. Catal. 2010, 352, 1870-1875.
- mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light, Fangzheng Su, Smitha C. Mathew, Grzegorz Lipner, Xianzhi Fu, Markus Antonietti, Siegfried Blechert, and Xinchen Wang, JACS, 2010, 132, 16299-16301.
- Aerobic Oxidative Coupling of Amines by Carbon Nitride Photocatalysis with Visible Light, Fangzheng Su, Smitha C. Mathew, Lennart Möhlmann, Markus Antonietti, XinchenWang, Siegfried Blechert, Angewandte Chemie 2011, 50, 657-660.
Project team and expertise
| Prof. Dr. Markus Antonietti (MPI-KGF) | Mesostructured materials, sol gel chemistry, SAXS |
| Prof. Dr. Siegfried Blechert (TU Berlin) | Homogeneous catalysis, organic synthesis, natural product synthesis |
| Prof. Dr. Thomas Braun (HU Berlin) | Mechanistic studies, coordination chemistry |
| Prof. Dr. Matthias Driess (TU Berlin) | Molecular main group and organometallic chemistry, precursors, NMR |
| Prof. Dr. Rainer Haag (FU Berlin) | Hyperbranched and dendritic polymers, homogeneous catalysis |
| Prof. Dr. Karola Rück-Braun (TU Berlin) | Organic synthesis, catalysis, peptide chemistry |
| Prof. Dr. Martin Kaupp (TU Berlin) | Quantum chemical calculations |
| Dr. Maria Schlangen (TU Berlin) | Mass spectrometry |
| Prof. Dr. Helmut Schwarz (TU Berlin) | Gas-phase ion and physical organic chemistry |
| Prof. Dr. Roderich Süssmuth (TU Berlin) | Biocatalysis, enzymatics, natural products, antibiotics, structure elucidation |
| Dr. Stephan Enthaler (TU Berlin) | Catalysts for oxidation and amination |
| Prof. Dr. Carl Christoph Tzschucke (FU Berlin) | Artificial photosystems for transition metal-catalysed redox reactions |


