B2 Structure-function analysis of oxygen tolerant hydrogenases
State of the art
The reversible cleavage of H2 into protons and electrons is a prevalent metabolic process in microorganisms, catalysed by two major classes of enzymes, the [NiFe]- and the [FeFe] hydrogenases. This project centres on the elucidation of the redox chemistry catalysed by oxygen-tolerant [NiFe] hydrogenases. Their active site is composed of an intricate complex of transition metals, forming a heterodinuclear Ni-Fe cofactor that is coupled with intramolecular electron and proton pathways.
Crystal structures and advanced spectroscopic analyses combined with molecular and biochemical tools have paved the way to a basic understanding of the reaction mechanism. It is generally accepted that the nickel-iron centre in the [NiFe] hydrogenases provides a free coordination site for binding hydrogen.
In the oxidised state of [NiFe] hydrogenases, this site is usually occupied by oxygen which has to be reductively removed prior to catalysis. The nature of the vacant site, of which subtle modifications may exist, provides one of the keys to understanding the catalytic properties of hydrogenases and offers potential targets for optimising the catalyst by genetic engineering.
Research goals
This research field focuses on the three [NiFe] hydrogenases in the ß-proteobacterium Ralstonia eutropha, which utilise the hydrogen-splitting reactions to perform different physiological functions, i.e. energy conservation, production of reducing equivalents and transcriptional regulation (hydrogen-sensing). Our major goal is to elucidate the molecular reaction mechanism of this group of [NiFe] hydrogenases, in particular the background for their oxygen tolerance and carbon monoxide insensitivity. Both features are crucial for the application of a hydrogen-activating catalyst. In general, oxygen may affect [NiFe] hydrogenases on three different levels, all of which are subjects of our research:
- posttranslational maturation
- catalytic activity
- regulation of gene transcription
Results/Achievements
Due to the excellent scientific environment of the UniCat cluster, a major goal of the 1st funding period could be accomplished, namely getting deeper insight into the molecular mechanism of O2 tolerance that is exhibited by certain [NiFe]-hydrogenases.
A multitude of genetic, biochemical, spectroscopic, and electrochemical data (ext. coll. F. Armstrong, K. Vincent, Oxford University, UK) was obtained during the current funding period [1-7]. In collaboration with F. Armstrong, a model was developed that provides the mechanistic background of O2 tolerance.
The results indicate that O2-tolerant hydrogenases actively remove the detrimental O2 rather than just preventing access of O2 to the catalytic centre as established in H2-sensing hydrogenases. In fact, it turned out that O2-tolerant biocatalysts are bifunctional, i.e. they convert H2 into protons and electrons and direct electrons to the catalytic centre in order to reduce O2 to water (see Fig. 2) [2, 5, 6].
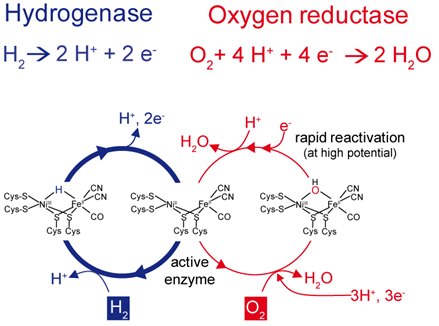
Fig. 2: Two different catalytic cycles compete in O2-tolerant [NiFe]-hydrogenases.
The vacant position between the nickel and iron atoms within the catalytic center can be occupied either by a hydride (left) or oxygen species (right). H2 conversion into protons and electrons represents the preferred pathway since O2 may be removed through reduction to water. This reaction requires 4 protons and 4 electrons, the latter of which have to be provided by the hydrogenase’s electron transfer chain by reverse electron flow.
An unusual iron-sulfur cluster coordinated by six Cys residues plays a crucial role in this process [6-8]. This cluster is located in the proximal position to the active site and is supposed to act as a molecular switch that directs the electron flow either from or towards the active site, depending on the nature of the bound substrate. This property is in accordance with the unique capability of the cluster to mediate two independent redox transitions at physiological potential [8].
The knowledge of the function of H2-converting biocatalysts in their native environment is necessary to evaluate the structural und functional integrity of the purified enzymes. Therefore we have undergone a successful comparative analysis of in vitro and in situ spectroscopic data obtained for the various O2-tolerant [NiFe]-hydrogenases of R. eutropha [1-4, 8, 9].
Most important publications
- Saggu, M., I. Zebger, M. Ludwig, O. Lenz, B. Friedrich, P. Hildebrandt, and F. Lendzian. Spectroscopic insights into the oxygen-tolerant membrane-associated [NiFe]-hydrogenase of Ralstonia eutropha H16.J. Biol. Chem. 284:16264–16276 (2009).
- Ludwig, M., J. A. Cracknell, K. A. Vincent, F. A. Armstrong, and O. Lenz. Oxygen-tolerant H2 oxidation by membrane-bound [NiFe]–hydrogenase of Ralstonia species: Coping with low-level H2 in air. J. Biol. Chem. 284:465-477 (2009).
- Wisitruangsakul, N., O. Lenz, M. Ludwig, B. Friedrich, F. Lendzian, P. Hildebrandt, I. Zebger. Monitoring catalysis of the membrane-bound hydrogenase from Ralstonia eutropha H16 by surface-enhanced infrared absorption spectroscopy. Angew. Chem. Int. Ed. Engl. 48:611-613 (2009).
- Saggu, M., C. Teutloff , M. Ludwig, M. Brecht, M. E. Pandelia, O. Lenz, B. Friedrich, W. Lubitz, P. Hildebrandt, F. Lendzian, and R. Bittl. Comparison of the membrane-bound [NiFe] hydrogenases from R. eutropha H16 and D. vulgaris Miyazaki F in the oxidized ready state by pulsed EPR. Phys. Chem. Chem. Phys. 12:2139-2148 (2010).
- Cracknell, J. A., A. F. Wait, O. Lenz, B. Friedrich, and F. A. Armstrong; A Kinetic and thermodynamic understanding of O2 tolerance in [NiFe]-Hydrogenases; Proc. Natl. Acad. Sci. U. S. A. 106:20681-20686 (2009).
- Lenz, O., M. Ludwig, T. Schubert, I. Bürstel, S. Ganskow, T. Goris, A. Schwarze, and B. Friedrich. H2 conversion in the presence of O2 as performed by the membrane-bound [NiFe]-hydrogenase of Ralstonia eutropha; Chem.Phys. Chem. 11:1107-1119 (2010).
- Fritsch, J, S. Löscher, O. Sanganas , E. Siebert, I. Zebger, M. Stein, M. Ludwig, A. L. De Lacey, H. Dau, B. Friedrich, O. Lenz, and M. Haumann. [NiFe]- and [FeS]-cofactors in the membrane-bound hydrogenase of Ralstonia eutropha investigated by X-ray absorption spectroscopy: insights into O2-tolerant H2-cleavage. Biochemistry DOI: 10.1021/bi200367u (2011).
- T. Goris , A. Wait , M. Saggu , J. Fritsch , N. Heidary , M. Stein , I. Zebger , F. Lendzian , F. Armstrong, B. Friedrich, and O. Lenz. A unique iron-sulfur cluster is crucial for oxygen tolerance of a [NiFe]-hydrogenase. Nat. Chem. Biol. 7:310-318 (2011)
- Horch, M., L. Lauterbach, M. Saggu, P. Hildebrandt, F. Lendzian, R. Bittl, O. Lenz, and I. Zebger. Probing the active site of an O2-tolerant NAD+-reducing [NiFe]-hydrogenase from Ralstonia eutropha H16 by in situ EPR and FTIR spectroscopy. Angew. Chem. Int. Ed. Engl. 49:8026-8029 (2010).
Project team and expertise
| Prof. Dr. Robert Bittl (FU Berlin) | EPR Spectroscopy |
| Prof. Dr. Holger Dau (FU Berlin) | X-ray spectroscopy, EXAFS |
| Prof. Dr. Holger Dobbek (HU Berlin) | Cofactor-containing enzymes |
| Prof. Dr. Bärbel Friedrich (HU Berlin) | Hydrogenases, physiology and biochemistry |
| Dr. Claudio Greco (HU Berlin) | Theoretical Bioinorganic Chemistry |
| Prof. Dr. Peter Hildebrandt (TU Berlin) | Vibrational spectroscopy, Photoreceptors |
| Prof. Dr. Martin Kaupp (TU Berlin) | Quantum chemical calculations |
| Dr. Oliver Lenz (HU Berlin) | Application of hydrogenases |
| Prof. Dr. Maria Andrea Mroginski (TU Berlin) | Theoretical approaches for spectroscopy |
Former team members | |
| PD Michael Haumann (FU Berlin) | Molecular biophysics, x-ray spectroscopy |