Models for formate dehydrogenase
Formate dehydrogenases (FDHs) catalyze the oxidation of formate to CO2. In the metal-containing FDH (see the cognate biological project E2-2), the active site of the enzyme is constituted by a Mo (or W) coordinated by two molybdopterin guanine dinucleotide cofactors and a SeCys (or Cys) residue.
No conclusive molecular mechanism for the catalytic process of this class of FDH has been established and biomimetic studies have been restricted so far to the synthesis of a few structural model complexes for the active site of Mo- and W-containing FDHs but none of them exhibited FDH activity.
Research goals
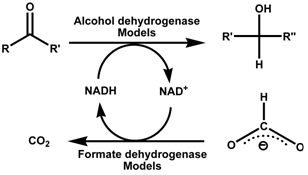
In this project we aim at designing efficient functional (bioinspired) model systems for the oxidation of formate. Thus we will mimic the active site of the Mo(W)-containing enzyme. Further efforts will also be made to couple the formate oxidation with aldehyde/ketone reduction via an alcohol dehydrogenase model.
Attempts will also be made to heterogenise the molecular pre-catalysts on metal oxide supports for comparative mechanistic studies and to improve work-up procedures.This may provide an efficient method for synthesizing the enantiomerically pure chiral alcohols, aiming at a viable alternative to the expensive use of the native enzymes.
