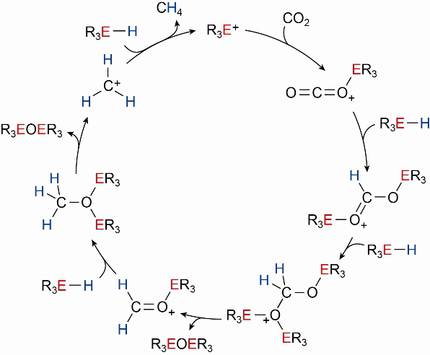
Chemical reduction of carbon dioxide to methane by main-group elements
The reduction of CO2 to intermediate oxidation levels, i.e. to formaldehyde (CH2O) or methanol (CH3OH), is significant for natural as well as industrial processes. To understand the mechanistic principles behind the involved consecutive reduction steps, model systems are required.
As an innovative approach in homogeneous catalysis, Si–H bonds have been recently identified to serve as potent hydride donors for the reduction of activated CO2. Using easily accessible main group element cations as catalysts, which can act as homogeneous as well as heterogeneous catalysts, the striking catalytic reduction of CO2 to CH4 with cheap silanes will be studied.
Research goals
Investigations on the kinetics of these reactions are carried out to unravel the rate-determining step and the overall mechanism of the consecutive processes (see Figure).
Computational studies will guide identification of the optimum hydride donor by predicting its hydride donor strength.
Heterogeneous hydrogenation catalysts will be developed to regenerate the hydride source from the corresponding oxygen-containing by-product.
In addition, novel “frustrated Lewis-pair” (FLP) systems will be employed in consecutive catalytic organic transformations such as hydrogenation of carbonyl compounds and subsequent C-C and C-N coupling to afford value-added products which are hitherto merely accessible by using precious metal catalysts.