Enzyme-mimicking and artificial hydrogenases
The cofactor of the [NiFe] hydrogenases is based on a [NiFe] core that is composed of (SCys)2Ni and Fe(CN)2(CO) subunits, bridged by two further SCys ligands. The centre is capable of binding H2 reversibly, affording a [NiIII-m2-H-FeII] moiety (see E3-1). None of the reported [NiFe]-containing model compounds exactly resembles the active site of the [NiFe]-hydrogenase and none of them has been shown to activate H2.
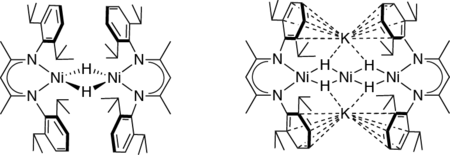
Since Ni is the redox-active component of the [NiFe] site during the catalytic process, we first pursued the synthesis of Ni complexes including bridging hydride ligands, yielding in the discovery of new types of Ni hydrides with Ni in the oxidation states +I and +II (see Figure).
Investigation of these complexes revealed that Ni hydrides can offer a versatile redox chemistry leading to remarkable structural and electronic properties, as well as to interesting reactivities similar to the biological template, i.e. [NiFe] hydrogenases. The trinuclear nickel compound shows hydrogenase-like activity (H2 activation):
Research goals
- Future goals are the evaluation of such trinuclear nickel compounds as potential hydrogenation catalyst and the mechanistic analysis of the catalytic process, which may also provide insight for understanding of the function of [NiFe] hydrogenases.
The mechanistic studies within the Ni3 system will be carried out using advanced EPR techniques, time-resolved spectroscopic analysis and theoretical analyses; investigations will be extended also to NiIII hydride chemistry. - Furthermore, the continuous endeavour to synthesise bio-inspired heterobimetallic Ni-containing complexes will be a central aim of this subproject.
These complexes including different metals and ligands may serve as structural and functional models for the active centre of the [NiFe] hydrogenase.
References
- S. Pfirrmann, C. Limberg, C. Herwig, C. Knispel, B. Braun, E. Bill, R. Stößer, J. Am. Chem. Soc. 2010, 132, 13684-13691.
- S. Pfirrmann, C. Limberg, B. Ziemer, Dalton Trans. 2008, 6689-6691.