Catalytic methods for the synthesis of novel non-natural amino acids
The synthesis of novel ncAA and their derivatives refers to three different target areas according to the specific objectives of their implementation into the target proteins, i.e. (i) fluorinated ncAAs, (ii) ncAAs with alkene moieties for metathesis methodologies, and (iii) photoactive or spectroscopic-tagged ncAAs. The latter will include the synthesis of ncAAs carrying reporter groups for IR spectroscopy such as a nitrile in cyano-Trp or an azide functionality in azido-Ala derivatives.
Research goals
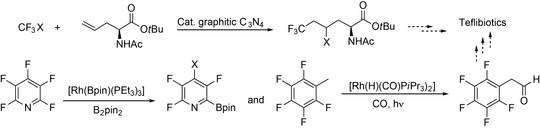
Fluorinated ncAA will be synthesized to generate fluorinated peptide antibiotics (“teflibiotics”). Their remarkable pharmacologic properties make fluorinated substrates particularly interesting in view of probing the ribosomal (RPS) and non-ribosomal peptide synthesys (NRPS) (E4-1) for generation of peptides with potentially enhanced bioactivity or enhanced pharmacological properties. Hence, novel catalytic routes to fluorinated building blocks will be developed.
For various ncAAs, orthogonal tRNA synthetase pairs have to be constructed for site-specific incorporation of the amino acid into the target protein. This task will be carried out in the research projects of E4. It is well documented that natural and orthogonal aminoacyl-tRNA synthetases display a wide substrate tolerance (i.e. substrate promiscuity) in activating structurally similar (analogs, surrogates) classes of amino acids. To expand the substrate tolerance sets of those each structurally and chemically non-related amino acids will be synthesized and exploited for guided evolution.