A Janus cobalt-based catalytic material for electro-splitting of water
Saioa Cobo, Jonathan Heidkamp, Pierre-André Jacques, Jennifer Fize, Vincent Fourmond, Laure Guetaz, Bruno Jousselme, Valentina Ivanova, Holger Dau, Serge Palacin, Marc Fontecave & Vincent Artero
Nature Materials 2012, 11 802- 807
The future of energy supply depends on innovative breakthroughs regarding the design of cheap, sustainable and efficient systems for the conversion and storage of renewable energy sources. The production of hydrogen through water splitting seems a promising and appealing solution.
We found that a robust nanoparticulate electrocatalytic material, H2–CoCat, can be electrochemically prepared from cobalt salts in a phosphate buffer. This material consists of metallic cobalt coated with a cobalt-oxo/hydroxo-phosphate layer in contact with the electrolyte and mediates H2 evolution from neutral aqueous buffer at modest overpotentials.
Remarkably, it can be converted on anodic equilibration into the previously described amorphous cobalt oxide film (O2–CoCat or CoPi) catalysing O2 evolution. The switch between the two catalytic forms is fully reversible and corresponds to a local interconversion between two morphologies and compositions at the surface of the electrode. After deposition, the noble-metal-free coating thus functions as a robust, bifunctional and switchable catalyst.
Room temperature femtosecond X-ray diffraction of photosystem II microcrystals
Jan Kern, Roberto Alonso-Mori, Julia Hellmich, Rosalie Tran, Johan Hattne, Hartawan Laksmono, Carina Glöckner, Nathaniel Echols, Raymond G. Sierra, Jonas Sellberg, Benedikt Lassalle-Kaiser, Richard J. Gildea, Pieter Glatzel, Ralf W. Grosse-Kunstleve, Matthew J. Latimer, Trevor A. McQueen, Dörte DiFiore, Alan R. Fry, Marc Messerschmidt, Alan Miahnahri, Donald W. Schafer, M. Marvin Seibert, Dimosthenis Sokaras, Tsu-Chien Weng, Petrus H. Zwart, William E. White, Paul D. Adams, Michael J. Bogan, Sébastien Boutet, Garth J. Williams, Johannes Messinger, Nicholas K. Sauter, Athina Zouni, Uwe Bergmann, Junko Yano, and Vittal K. Yachandra
PNAS, 2012, 109 (25), 9721-9726
Most of the dioxygen on earth is generated by the oxidation of water by photosystem II (PS II) using light from the sun. This light-driven, four-photon reaction is catalyzed by the Mn4CaO5 cluster located at the lumenal side of PS II. Various X-ray studies have been carried out at cryogenic temperatures to understand the intermediate steps involved in the water oxidation mechanism. ...
Mesoporous Nitrogen-Doped Carbon for the Electrocatalytic Synthesis of Hydrogen Peroxide
Tim-Patrick Fellinger, Frédéric Hasché, Peter Strasser and Markus Antonietti
J. Am. Chem. Soc., 2012, 134, 4072–4075
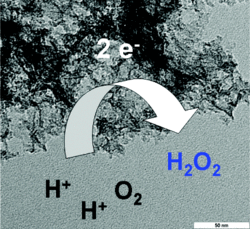
Mesoporous nitrogen-doped carbon derived from the ionic liquid N-butyl-3-methylpyridinium dicyanamide is a highly active, cheap, and selective metal-free catalyst for the electrochemical synthesis of hydrogen peroxide that has the potential for use in a safe, sustainable, and cheap flow-reactor-based method for H2O2 production.
Size-Dependent Morphology of Dealloyed Bimetallic Catalysts: Linking the Nano to the Macro Scale
Mehtap Oezaslan, Marc Heggen, and Peter Strasser
J. Am. Chem. Soc., 2012, 134, 514–524
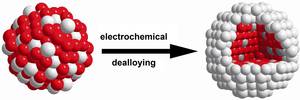
Chemical dealloying of Pt binary alloy precursors has emerged as a novel and important preparation process for highly active fuel cell catalysts. Dealloying is a selective (electro)chemical leaching of a less noble metal M from a M rich Pt alloy precursor material and has
been a familiar subject of macroscale corrosion technology for decades.
The atomic processes occurring during the dealloying of nanoscale materials, however, are virtually unexplored and hence poorly understood. Here, we have investigated how the morphology and intraparticle composition depend on the particle size of dealloyed Pt–Co and Pt–Cu alloy nanoparticle precursor catalysts.
To examine the size–morphology–composition relation, we used a combination of high-resolutionscanning transmission electron microscopy (STEM), transmission electron microscopy (TEM), electron energy loss (EEL) spectroscopy, energy-dispersive X-ray spectroscopy (EDS), and surface-sensitive cycling voltammetry.
Our results indicate the existence of three distinctly different size-dependent morphology regimes in dealloyed Pt–Co and Pt–Cu particle ensembles: (i) The arrangement of Pt shell surrounding a single alloy core (“single core–shell nanoparticles”) is exclusively formed by dealloying of particles below a characteristic diameter dmultiple cores of 10–15 nm. (ii) Above dmultiple cores, nonporous bimetallic core–shell particles dominate and show structures with irregular shaped multiple Co/Cu rich cores (“multiple cores–shell nanoparticles”). (iii) Above the second characteristic diameter dpores of about 30 nm, the dealloyed Pt–Co and Pt–Cu particles start to show surface pits and nanoscale pores next to multiple Co/Cu rich cores.
This structure prevails up to macroscopic bulklike dealloyed particles with diameter of more than 100 nm. The size–morphology–composition relationships link the nano to the macro scale and provide an insight into the existing material gap of dealloyed nanoparticles and highly porous bulklike bimetallic particles in corrosion science.
This paper has been highlighted in JACS by Christen Brownlee.