B1 Photosynthetic water oxidation and bioelectronic devices
Photosynthetic water oxidation
Exploiting solar energy, plants and cyanobacteria convert water and carbon dioxide into the energy-rich carbohydrates that ultimately fuel life on earth. We focus on water oxidation at the pentanuclear Mn4Ca complex bound to photosystem II (PSII), a membrane-intrinsic protein complex. Aside from its fundamental importance in the biosphere, catalysis of water oxidation is of high interest because of its pivotal role in envisioned systems for large-scale synthesis of non-fossil fuels.
Our central research targets are:
- Essential contributions to elucidation of the catalytic mechanism of biological water oxidation.
- Bridging the gap toward homogeneous and heterogeneous catalysis by comparative investigations on biomimetic catalysts.
Instrumental and methodical developments are an important part of the experimental program, which is pursued in conjunction with researchers in the fields B2 and A5 (and others).
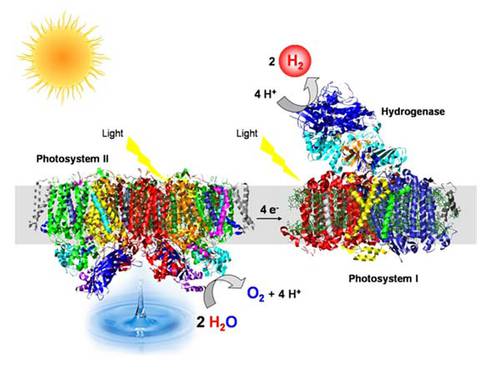
Bioelectronic devices for biofuel cells and light-driven hydrogen production
Research on photosynthetic water oxidation and hydrogenases is linked by an exciting long-term prospect: the efficient coupling of both processes with the objective of producing hydrogen from light and water.
Central research target is the direct coupling of O2-tolerant [NiFe]-hydrogenases, which are investigated in Research Field B2, to peripheral subunits of photosystem I (PSI) by using different strategies. The efficiency of these devices crucially depends on the tight electronic coupling of the components, which will be optimised by genetic engineering of the respective coupling partners and subsequent biochemical and spectroscopic analysis by UniCat scientists and collaborators.
Selected results
- Basic insights in reaction cycle, performance level, and thermodynamic limits of photosynthetic water oxidation resulted from time-resolved X-ray absorption measurements at elevated O2 partial pressure [1] and recombination-fluorescence experiments [2].
- By structural biology (protein crystallography), insights in the role of quinones, lipids, channels and chloride [3], and the mode of herbicide inhibition [4] was obtained.
- Properties of light-absorbing pigments in the photosystems of oxygenic photosynthesis were investigated employing novel approaches, namely polarized Raman spectroscopy on single PSII crystals [5] and single-molecule experiments on PSI [6].
- A new experiment for fluorescence-detected X-ray absorption spectroscopy on metalloproteins and synthetic systems was implemented at the Berlin synchrotron radiation facility (BESSY) and meanwhile has been employed in numerous investigation of UniCat researchers, national and international partners [7, 8].
- First steps have been taken successfully toward addressing electronic and geometric structure of (bio)catalysts in a comprehensive X-ray approach involving multiple X-ray edge analysis and high-resolution emission spectroscopy.
- A bridge toward synthetic catalysts was established conceptually [9] and in investigations on water-oxidizing transition metal oxides. A specific calcium-manganese oxide was identified as being the closest functional and structural mimic of the photosynthetic Mn4Ca complex synthesized so far [8].
- Improved mechanistic understanding of biosynthesis and catalysis of O2-tolerant hydrogenases in conjunction with the possibility to purify the enzymes in large amounts at high quality has provided the basis for new potential applications of these complex biocatalysts (see Research Field B2).
- We could show for the first time that biocatalytic H2 production is possible in the presence of O2 (albeit at lower turnover rates compared to O2-sensitive hydrogenases under strictly anaerobic conditions) [10].
- Because of their capability to catalyse both H2 consumption and H2 production under aerobic conditions, O2-tolerant hydrogenases received great attention as attractive catalysts for biotechnological application [11]. This includes enzymatic fuel cells as well as light-driven H2-production by hybrid protein complexes consisting of a hydrogenase directly coupled to PSI. By exploiting natural protein-protein interactions, we have constructed a PSI-hydrogenase fusion complex that shows the highest H2 production rate compared to published in vitro systems [12, 13].
Most important publications
M. Haumann, A. Grundmeier, I. Zaharieva, H. Dau, Photosynthetic water oxidation at elevated dioxygen partial pressure monitored by time-resolved X-ray absorption measurements, Proc. Natl. Acad. Sci. USA 105 (2008) 17384-17389.
I. Zaharieva, J.M. Wichmann, H. Dau, Thermodynamic limitations of photosynthetic water oxidation at high proton concentrations, J. Biol. Chem. 286 (2011) 18222-18228.
A. Guskov, J. Kern, A. Gabdulkhakov, M. Broser, A. Zouni, W. Saenger, Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride, Nat Struct. Mol. Biol. 16 (2009) 334-342.
M. Broser, C. Glöckner, A. Gabdulkhakov, A. Guskov, J. Buchta, J. Kern, F. Müh, H. Dau, W. Saenger, A. Zouni, Structural basis of cyanobacterial photosystem II inhibition by the herbicide terbutryn, J. Biol. Chem. 286 (2011) 15964-15972.
K. Brose, A. Zouni, M. Broser, F. Muh, J. Maultzsch, Polarised Raman measurements on the core complex of crystallised photosystem II, Phys. Status Solidi B 246 (2009) 2813-2816.
M. Brecht, V. Radics, J.B. Nieder, R. Bittl, Protein dynamics-induced variation of excitation energy transfer pathways, Proc. Natl. Acad. Sci. U S A 106 (2009) 11857-61.
F. F. Pfaff, S. Kundu, M. Risch, S. Pandian, F. Heims, I. Pryjomska-Ray, P. Haack, R. Metzinger, E. Bill, H. Dau, P. Comba, K. Ray; An oxocobalt(IV) complex stabilized by Lewis acid interactions with scandium(III) ions; Angew. Chem. Int. Ed. 50 (2011) 1711-1715.
I. Zaharieva, M.M. Najafpour, M. Wiechert, M. Haumann, P. Kurz, H. Dau, Synthetic manganese-calcium oxides mimic the water-oxidizing complex of photosynthesis functionally and structurally, Energy Environ. Sci. 4 (2011) 2400-2408.
H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan, P. Strasser, The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis, ChemCatChem 2 (2010) 724-761.
Goldet, G., A. F. Wait, J. A. Cracknell, K. A. Vincent, M. Ludwig, O. Lenz, B. Friedrich, F. A. and Armstrong. Hydrogen production under aerobic conditions by membrane-bound hydrogenases from Ralstonia species. J. Am. Chem. Soc. 130 (2008) 11106-13.
Friedrich, B., J. Fritsch & O. Lenz. Oxygen-tolerant hydrogenases in hydrogen-based technologies. Curr. Opin. Biotechnol. 22 (2011) 358-364.
Schwarze, A., M. J. Kopczak, M. Rögner & O. Lenz. Requirements for construction of a functional hybrid complex of photosystem I and [NiFe]-hydrogenase. Appl. Environ. Microbiol. 76 (2010) 2641-2651.
Krassen, H., A. Schwarze, B. Friedrich, K. Ataka, O. Lenz & Joachim Heberle. Photosynthetic hydrogen production by a hybrid complex of photosystem I and [NiFe]-hydrogenase. ACS Nano. 3 (2009) 4055-4061.
Project team and expertise
| Prof. Dr. Robert Bittl (FU Berlin) | advanced EPR spectroscopy, single-molecule experiments |
| Prof. Dr. Holger Dau (FU Berlin) | mechanistic investigations on water oxidation, X-ray spectroscopy |
| Dr. Anna Fischer (TU Berlin) | |
| Dr. Oliver Lenz (HU Berlin) | biochemistry and molecular biology of hydrogenases |
| Prof. Dr. Athina Zouni (TU Berlin) | protein purification, protein crystallography, PSII structure |
Former team members | |
| Prof. Dr. Peter Hildebrandt (TU Berlin) | Vibrational spectroscopy |
| Prof. Dr. Martin Kaupp (TU Berlin) | Quantum chemical calculations |
| Prof. Dr. Maria Mroginski (TU Berlin) | Quantum chemical calculations |
| Prof. Dr. Christian Thomsen (TU Berlin) | Vibrational spectroscopy |
| Prof. Dr. Ulla Wollenberger (Uni Potsdam) | Electrochemistry |