Very Important Paper in Angewandte Chemie by Prof. Dau and Driess
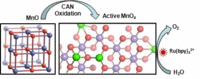
Yes, we CAN: Partial oxidation of inactive MnO nanoparticles by CeIV as oxidant gives active MnOx catalysts that are suitable for effective photochemical and electrochemical water oxidation. The active MnOx catalyst contains mixed-valent MnII, MnIII, and MnIV species (see picture; green and violet) interconnected through oxido bridges (red) with defects and disorders. MnOx is analogous to calcium–manganese oxide systems where the calcium sites are replaced by MnII or MnIII ions.
The unexpected facile conversion of catalytically inactive nanostructured MnO precursors into active amorphous MnOx catalysts is report for the first time for effective water oxidation by using ceric ammonium nitrate (CAN) as an oxidant (“corrosion agent”). The effective photochemical water oxidation of the active catalysts has been carried out in the presence of [Ru(bpy)3]2+ as a photosensitizer.
Structural characterization of the active MnOx catalyst by XAS revealed the presence of MnII, MnIII, and MnIV sites. A series of defects and structural disorder in the di-µ-oxido-bridged structure makes the catalyst analogous to calcium-manganese oxide systems, but the calcium sites are occupied by MnII, or MnIII ions.
In other words, the structural motif of the active MnOx catalyst is close to biomimetic water oxidation catalyst systems, which explains its efficient catalytic activity in water oxidation.
This paper of Holger Dau and Matthias Driess has been selected as a very important paper in Angewandte Chemie.
Angew. Chem. Int. Ed. 2013; DOI: anie.201307543
Published online 31 October 2013.
See also Research\Selected Research Highlights.