Artificial proteins with novel properties
The use of non-canonical amino acids in protein engineering gained much attention in recent years. Genetic code engineering is based on in vivo sense-codon reassignment, whereby synthetic amino acids are translated into protein sequences in residue-specific manner. In this way proteins are chemically enriched in a controlled manner without need for DNA mutagenesis. But until now, only one type of non-canonical amino acids could be introduced into protein sequences.
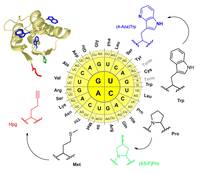
The group of Prof. Budisa could now demonstrate that it is possible to introduce three types of non-canonical amino acids into a protein in a single expression experiment. By this, a blue fluorescent chromophore, a stabilizing amino acid and a reactive handle could be incorporated into a protein. The latter one allows further bio-orthogonal modification of the biopolymer. These results published online on June, 24th 2010 provide a new tool for protein engineering in general but also for related research fields like enzyme evolution, cell biology, biophysics and (bio)material sciences.
Sandra Lepthien, Lars Merkel, Nediljko Budisa: In vivo double and triple labeling of proteins using synthetic amino acids. Angewandte Chemie Int. Ed., Vol. 49, p 1- 6, 2010.
For more information:
Prof. Dr. Nediljko Budisa
Institut für Chemie
Technische Universität Berlin
Tel: (030) 314-23 661
E-Mail: budisa(at)biocat.tu-berlin.de