Metal-free hydrogenation catalysis
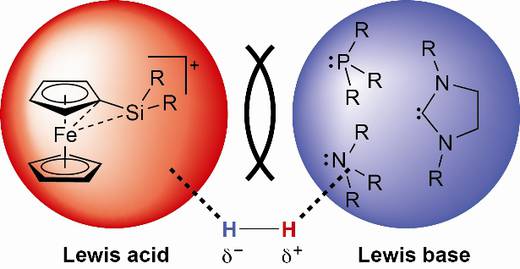
Recently, a novel and promising approach towards the activation and utilisation of H2 in catalysis has emerged from studies in main group chemistry. Unique reactivity results from the use of frustrated lewis pairs (FLPs) for heterolytic dihydrogen activation enabling hydrogenation catalysis at atmospheric pressure and ambient temperature without involving precious metals.
Our efforts in main-group chemistry and organic synthesis will be devoted to apply novel innovative silicon- and germanium-based FLPs capable for H2 activation and catalytic hydrogenation.
Research goals
- Versatile and isolable N-heterocyclic, ylide-like silylenes and germylenes will be exploited which are capable of heterolytic bond activation. With the aid of co-substrates, the systems will be modified to operate reversibly, specifically directed to the catalytic hydrogenation of polar C=X bonds (X = N, O). This concept will be guided by theoretical predictions.
- Likewise, “tamed” ferrocene-substituted silylium ions which have recently been introduced as a novel family of extremely potent Lewis acids will be used as alternatives to B(C6F5)3 in the formation of FLPs for H2 activation.
- Chiral FLPs will be used for enantioselective hydrogenation and transfer hydrogenation processes and studied mechanistically.