Biocatalytic activation of dioxygen and peroxides
We still need to gain a better understanding of elementary steps in immobilised enzymes that are able to activate dioxygen and H2O2. These studies aim at constituting the basis for a knowledge-based design of enzymatic hybrid devices to be transferred to biotechnological applications of electrogenic reduction in biofuel cells (see E2-2) and signal generation in biosensors.
This project starts from natural peroxygenases and oxygenases and it will be extended to the design of artificial enzyme systems. The research builds upon achievements of the UniCat cluster in synthesis of novel conductive support materials, immobilisation strategies for enzymes and their electrochemical characterisation, and the development of surface-sensitive spectroelectrochemical techniques.
Research goals
- The central goal is the design of artificial enzymes on the basis of minimum catalytically competent subunits of natural cofactor-protein complexes. These subunits are obtained by truncating the polypeptide chains of the parent heme proteins such as c-type cytochromes, cytochromes P-450, plant peroxidases and peroxygenases using cell-free protein synthesis and structure-based directed evolution, recombinant protein production and screening.
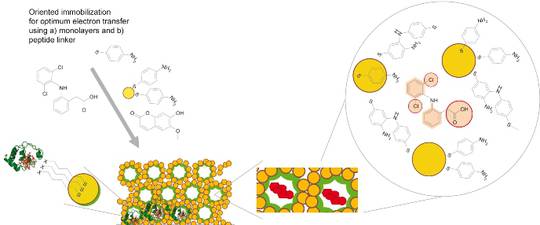
- Effective bioelectrocatalysis will be achieved by coupling these so called “mini-enzymes” to functionalised nanostructured conducting materials including arrays of nanoparticles, biocompatible coatings such as self-assembled monolayers (SAMs) of metal-binding peptides and 2- and 3-D polymer scaffolds.
- (Bio)catalysts obtained by these strategies with respect to their performance and their respective catalytic mechanism will be characterised by biochemical assays, SERR and surface-enhanced infrared absorptions (SEIRA) spectroscopy, and electrochemical methods.