Biological hydrogen conversion
The biocatalytic splitting of molecular hydrogen into protons and electrons as well as the reverse reaction, the formation of H2 through proton reduction, are widespread traits in the microbial world. These processes are catalysed by complex metalloenzymes denoted as hydrogenases.
The Berlin scientists involved in hydrogenase research are experts on a particular subgroup of H2-converting biocatalysts, the so-called O2-tolerant hydrogenases, which harbor a [NiFe] catalytic centre.
In contrast to the vast majority of hydrogenases, which are immediately inactivated upon exposure to dioxygen, O2-tolerant hydrogenases sustain H2 cycling even at ambient O2 concentration. In fact, it turned out that these particular biocatalysts are bi-functional, i.e. they convert H2 into protons and electrons and are capable in directing electrons to the catalytic centre in order to reduce O2 completely to water [1-3].
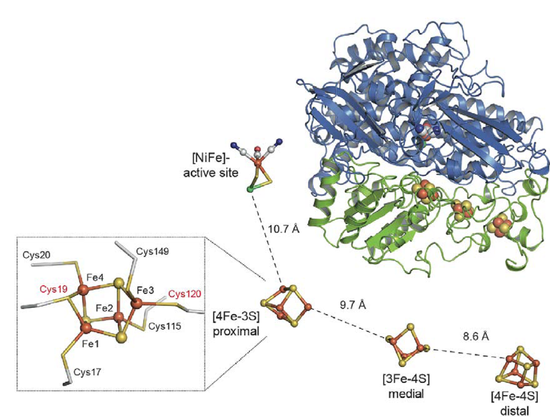
The recently solved crystal structure provides first insights into the atomic basis of O2 tolerance and has uncovered an unprecedented [4Fe-3S] cluster coordinated by six Cys residues, which seems to act as a molecular switch directing the electron flow either from or towards the active site, depending on the nature of the bound substrate [2; 4].
Research goals
- The crystal structure provides an excellent starting point for a comprehensive extraction of structural and mechanistic data obtained from IR and EPR spectroscopy in combination with electrochemistry and theoretical methods (QM/MM).
- We are now trying to obtain diffracting crystals from genetically engineered hydrogenase derivatives containing specific amino acid exchanges close to the [FeS] centres. Based on the structural information obtained from these enzyme variants, we will elucidate the unique electronic properties of the various cofactors in this biocatalyst.
- We aim at a comprehensive understanding of the catalytic mechanism(s) of O2-tolerant hydrogenases, particularly their O2 reduction capacity. Mass spectrometry will be employed to investigate the fate of 18O-labelled O2 during H2 conversion under aerobic conditions.
- An additional goal will be the elucidation of the biosynthesis and assembly of the catalytic cofactor(s) of [NiFe]-hydrogenases.
References
- J. A. Cracknell, A. F. Wait, O. Lenz, B. Friedrich, F. A. Armstrong; A Kinetic and thermodynamic understanding of O2 tolerance in [NiFe]-Hydrogenases; Proc. Natl. Acad. Sci. USA 2009, 106,20681-20686.
- T. Goris, A. Wait, M. Saggu, J. Fritsch, N. Heidary, M. Stein, I. Zebger, F. Lendzian, F. Armstrong, B. Friedrich, O. Lenz; A unique iron-sulfur cluster is crucial for oxygen tolerance of a [NiFe]-hydrogenase; Nat. Chem. Biol. 2011, 7, 310-318.
- O. Lenz, M. Ludwig, T. Schubert, I. Bürstel, S. Ganskow, T. Goris, A. Schwarze, B. Friedrich, ChemPhysChem. 2010, 11,1107.
- J. Fritsch, P. Scheerer, S. Frielingsdorf, S. Kroschinsky, B. Friedrich, O. Lenz, C. M. Spahn; The crystal structure of an oxygen-tolerant hydrogenase unmasks a novel iron-sulphur centre; Nature, 2011, published online 16 October 2011; DOI 10.1038/nature10505.