Unicat researchers provide new insights into the nature and function of hydrogen-oxidizing enzymes
11 March 2016
Hydrogenases are enzymes that split molecular hydrogen into protons and electrons, which are conversely used to form hydrogen. Whether or not a particular hydrogenase is oxygen-tolerant is determined by its 3D-structure. The nature of the metal-containing active site and several metal clusters appear to play a significant role in this regard, as does the number and size of gas tunnels. This is the conclusion drawn by Dr. Patrick Scheerer from Charité – Universitätsmedizin Berlin and Dr. Oliver Lenz from Technische Universität Berlin. Both collaborated on this project under the auspices of the Unifying Concepts in Catalysis (UniCat) Cluster of Excellence. Results from their study have been published in the current issue of the journal Angewandte Chemie.
Hydrogen is generally considered to be the ‘energy carrier of the future’. The combustion of hydrogen and oxygen (Knallgas reaction) releases a considerable amount of energy (286 kJ/mol). Certain anaerobic microorganisms, i.e. microorganisms that thrive in the absence of oxygen, use hydrogen as a source of energy. They use different hydrogenases to oxidize molecular hydrogen, thus releasing its chemical energy. There also are other microorganisms which are capable of releasing excess energy in the form of hydrogen, and which use a different type of hydrogenase to do so.
“A characteristic feature common to all hydrogenases is oxygen sensitivity. Exposure to oxygen can result in irreversible damage to the enzyme's active site,” says Dr. Patrick Scheerer of Charité's Institute of Medical Physics and Biophysics, who heads the working group. He adds: “There are exceptions to this rule, such as certain aerobic, hydrogen-oxidizing bacteria that are sometimes referred to as Knallgas bacteria. These bacteria live in aerobic conditions, but have adapted in a way such that they are able to use hydrogen as a source of energy.” The study set out to investigate possible reasons for why the hydrogenase of Knallgas bacteria shows an increased resistance to oxygen.
The photosynthesis-driven production of biological hydrogen using light energy and water is one area where a detailed breakdown of the characteristics of oxygen-tolerant hydrogenases is of great interest, and offers new perspectives for the use of these enzymes. The benefit of enzymatic biofuel cells to generate electricity is another area where oxygen-tolerant hydrogenases play a hugely significant role.
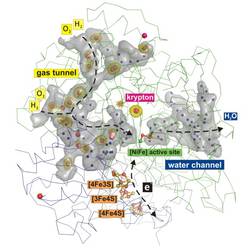
The researchers used different methods to study the protein structure of these enzymes, including the noble gas derivatization of protein crystals and protein X-ray crystallography. They also analyzed the routes, length and size of the hydrophobic (water-repellent) gas tunnels that allow substrates to reach the active site deep inside the enzyme. Based on their calculations, the researchers also developed models to compare the structures of oxygen-tolerant and oxygen-sensitive hydrogenases. “Our results show considerable differences between the different groups of hydrogenases in terms of the number and size of their hydrophobic gas tunnels,” explains Jacqueline Kalms, the study's first author, who goes on to add: “It is possible that these differences determine the exact nature of that remarkable property – oxygen tolerance.”
Original publication
Krypton Derivatization of an O2 -Tolerant Membrane-Bound [NiFe] Hydrogenase Reveals a Hydrophobic Tunnel Network for Gas Transport. Kalms J, Schmidt A, Frielingsdorf S, van der Linden P, von Stetten D, Lenz O, Carpentier P, Scheerer P. Angew. Chem. Int. Ed. 2016 Feb 23. DOI: 10.1002/anie.201508976. [Epub ahead of print] PMID:26913499.
Contact
Dr. Patrick Scheerer
Institut für Medizinische Physik und Biophysik
AG Proteinstrukturanalyse und Signaltransduktion
Charité - Universitätsmedizin Berlin
Phone: +49 (0)30 450 524 178
Dr. Julia Biederlack
Corporate Communications
Charité – Universitätsmedizin Berlin